Charge Density Definition Chemistry
This definition of polarization density as a dipole moment per unit volume is widely adopted though in some cases it can lead to ambiguities and paradoxes. In electromagnetism charge density is the amount of electric charge per unit length surface area or volume.
Illustrated Glossary Of Organic Chemistry Term
It is a low-density compound.

. Helium balloons float because the density of the helium is lower than the density of air. A coulomb is a type of unit of electric charge and is equivalent to one ampere being steadily transported per one second. The structure of carbon charcoal shows a large surface area.
To show these two physical properties a surfactant must have. Volume charge density symbolized by the Greek letter ρ is the quantity of charge per unit volume measured in the SI system in coulombs per cubic meter Cm 3 at any point in a volume. 16 x 10 -19 C or 16 x 10 -19 C Explanation.
One of the most common uses of density is in how different materials interact when mixed together. When an object has a positive charge it means that it has more protons than electrons. A formal charge FC.
Wood floats in water because it has a lower density while an anchor sinks because the metal has a higher density. Types of electric charge. Two kinds of electric charges are there.
The formal charge is the difference between an atoms number of valence electrons in its neutral free state and the number allocated to that atom in a. Due to polarization the positive. 1 elementary charge is equal to.
The high surface area of charcoal and high porosity enhances the contamination of charcoal by incidental contact with dust and soil. Electrical charge resides in electrons and protons and the smallest charge that a body can have is the charge of one electron or proton. Surfactants are substances that create self-assembled molecular clusters called micelles in a solution water or oil phase and adsorb to the interface between a solution and a different phase gasessolids.
Or q is the charge assigned to an atom in a molecule in the covalent view of bonding assuming that electrons in all chemical bonds are shared equally between atoms regardless of relative electronegativity. It readily absorbs moisture. Charcoal shows low mechanical strength properties.
A statcoulomb is a unit of electric charge in the centimeter-gram-second system of units cgs and Gaussian units. 1602 x 10-19 coulombs. 480320425 x 10-10 statcoulombs.
When an object has a negative charge it means that it has more electrons than protons. 1 The net charge appearing as a result of polarization is called bound charge and denoted Q b displaystyle Q_b. Other expressions Let a volume d V be isolated inside the dielectric.
Nakama in Cosmetic Science and Technology 2017 152 Characteristics and Classification of Surfactants. Surface charge density σ is the quantity of charge per unit area measured in. Charcoal acts as a good absorbent.

What Is Effective Nuclear Charge And How To Calculate It Slater S Rules

Chemical Reaction Flashcard 9th Grade Science Chemical Reactions Science Flashcards Chemical Bond
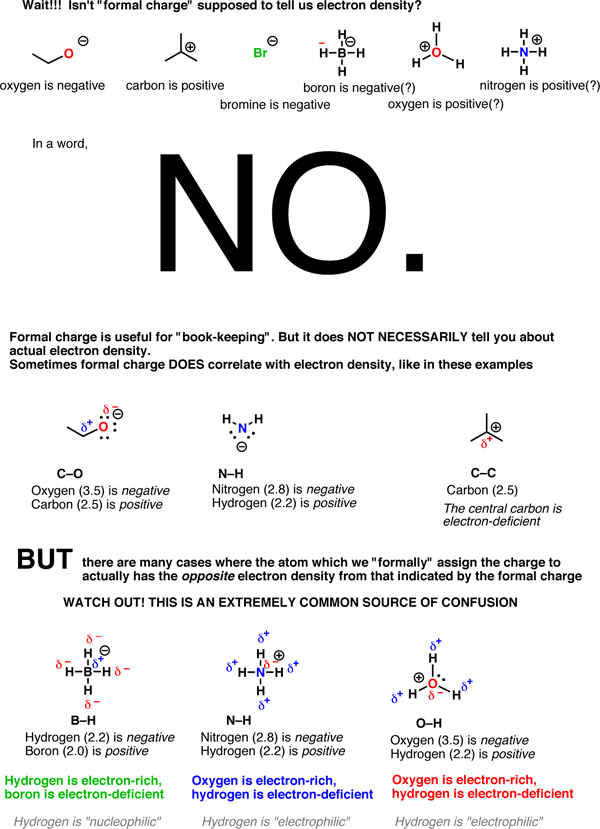
How To Use Electronegativity To Determine Electron Density And Why Not To Trust Formal Charge Master Organic Chemistry

Comments
Post a Comment